What is the formula to calculate the radius of an orbit of the atom and velocity of the specific shell of the atom.
Atomic radius is of order 10^-8 cm and nuclear radius is of order 10^-13. calculate what fraction of atom is occupied by nucleus? | Socratic
Derive a formula for radius of the stable orbit of hydrogen atom on the basis of Bohr model. Prove that in hydrogen - Sarthaks eConnect | Largest Online Education Community

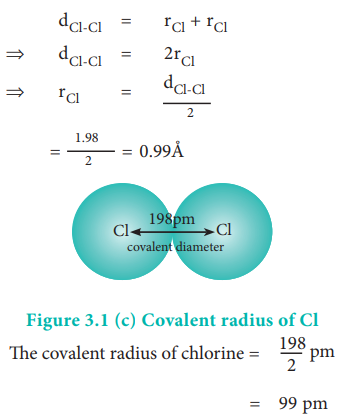
What do you mean by covalent radius? from Chemistry Classification of Elements and Periodicity in Properties Class 11 Karnataka Board

Atomic radius and ionic radius of F(g) and F(g)^- are 72 and 136 pm prespectivley. Calculate the ratio and percentage increase in terms of volume during formation of F(g)^(-) form F(g) .

The value of covalent bonf length of Si - C is 1.93 A . Covalent radius of carbon atom is 0.77 A . Calculate the covalent radius of silicon atom.

Calculate the radius of second Bohr orbit in hydrogen atom from the given data.Mass of electron = 9.1 × 10^-31Kg Charge on the electron = 1.6 × 10^-19C Planck's constant = 6.63 ×
Welcome to Chem Zipper.com......: How to calculate percentage (%) ionic character in covalent compounds?