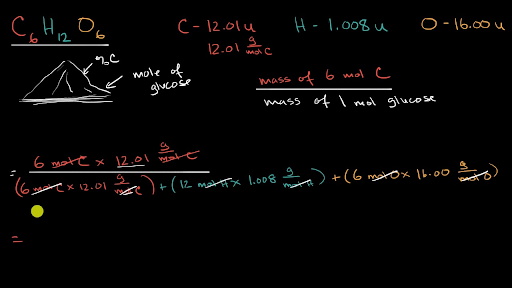Question Video: Calculating the Percent by Mass of the Active Ingredient in an Anti-inflammatory Ointment | Nagwa
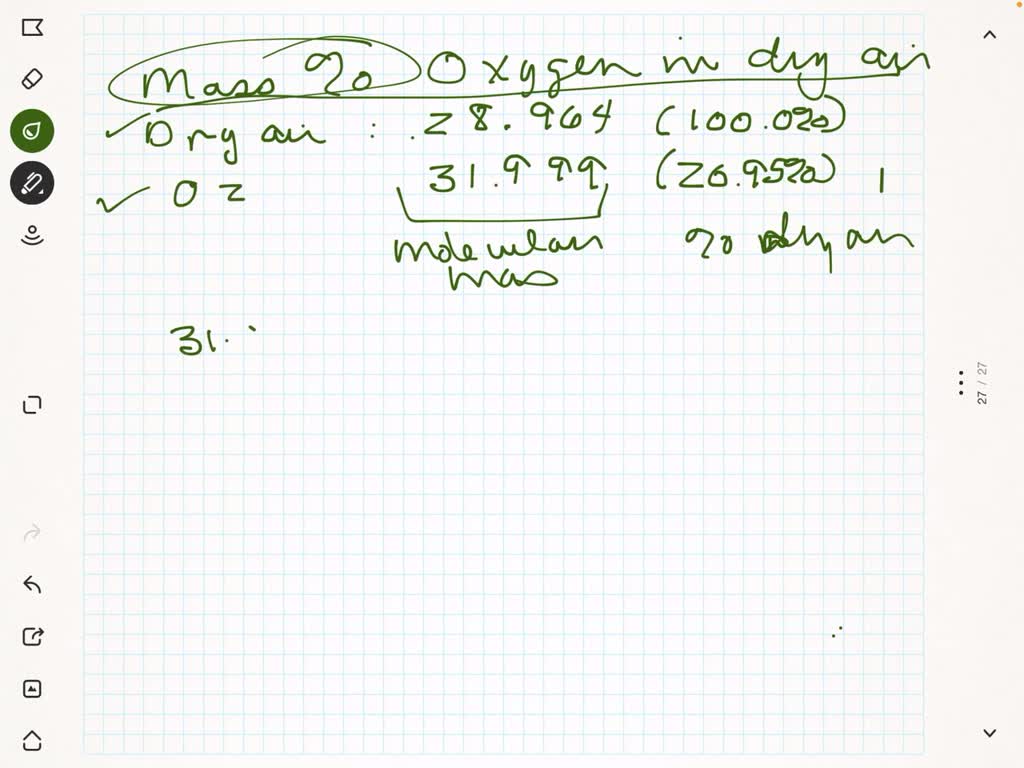
SOLVED: Part B Calculate the mass percentage of oxygen in dry air Express your answer with the appropriate units. View Available Hint(s) Value Units Submit

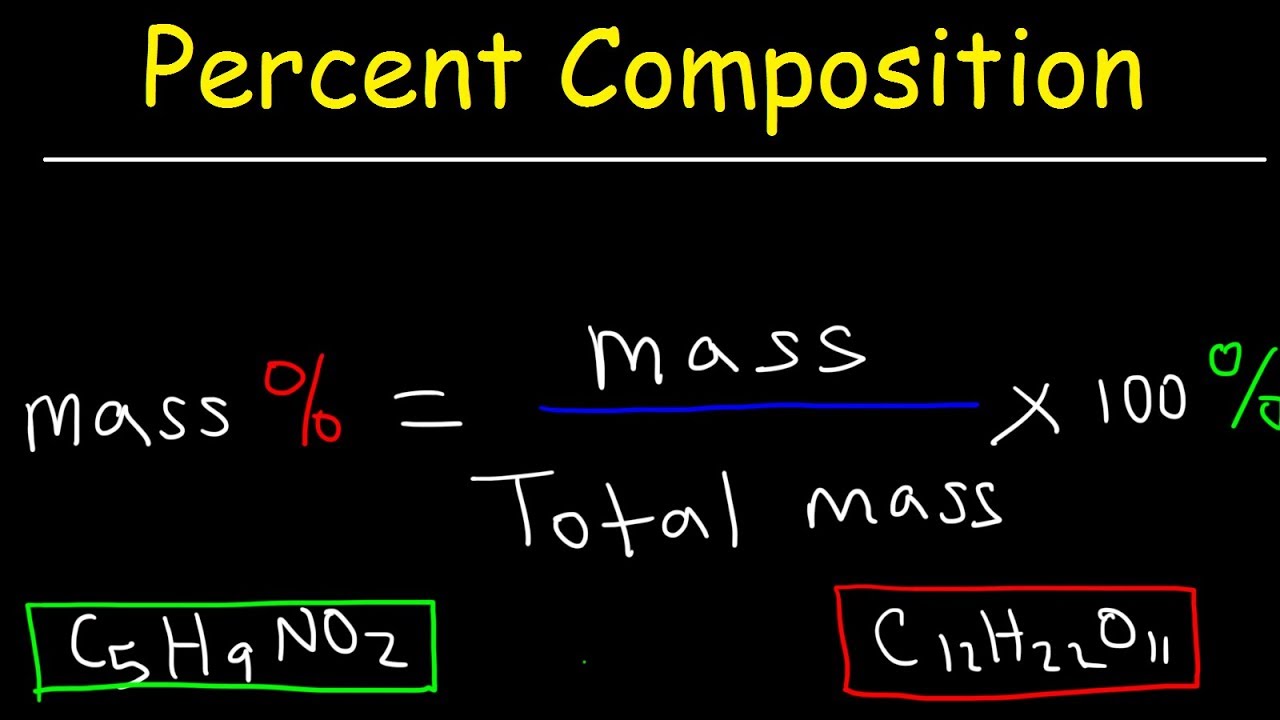
How to Calculate Mass Percent of Element in Compound Examples, Practice Problem, Explained, Shortcut - YouTube


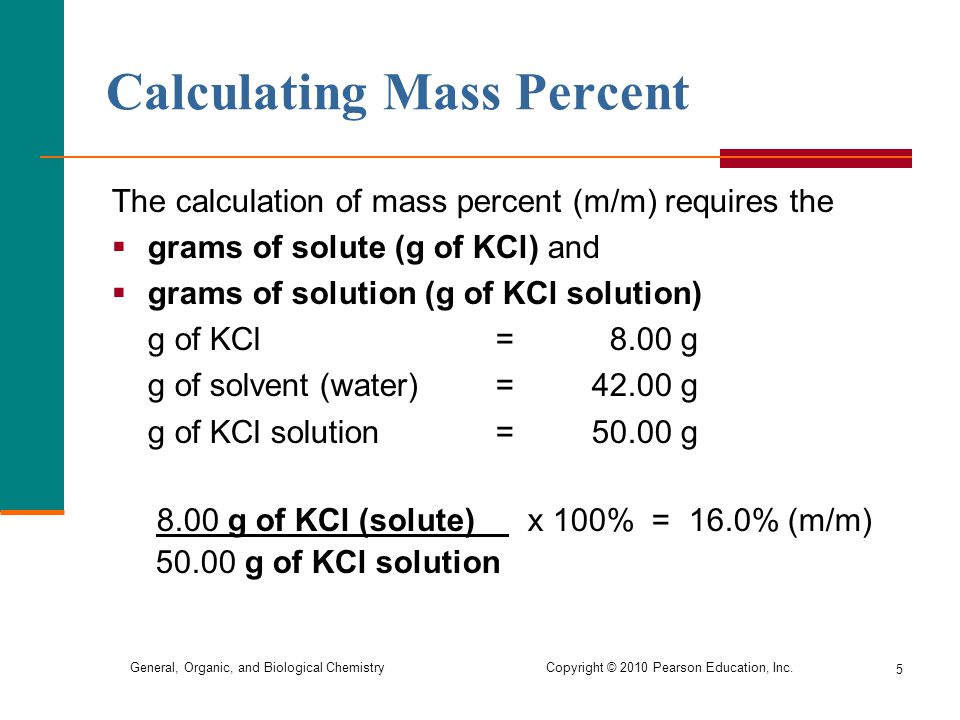






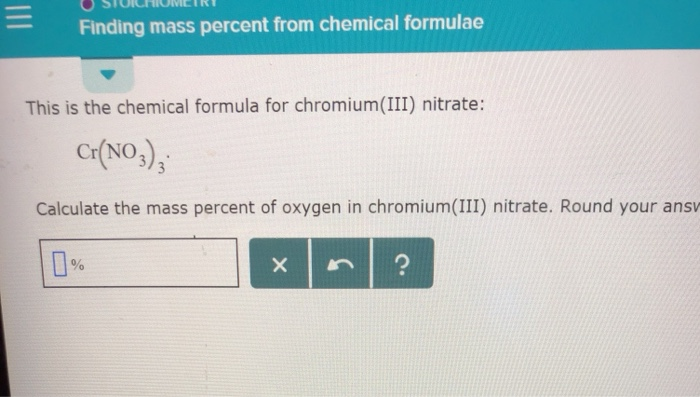


:max_bytes(150000):strip_icc()/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)



:max_bytes(150000):strip_icc()/Potassium-ferricyanide-58a279693df78c475811ba19.jpg)
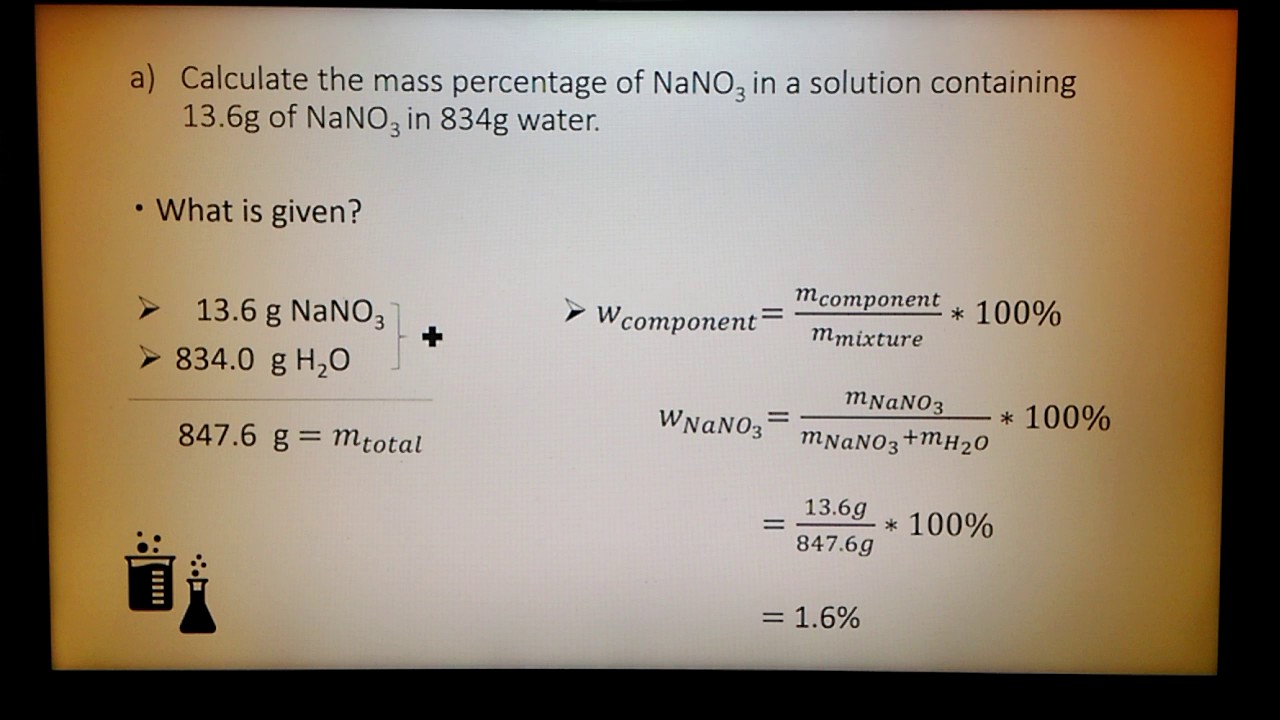
:max_bytes(150000):strip_icc()/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)